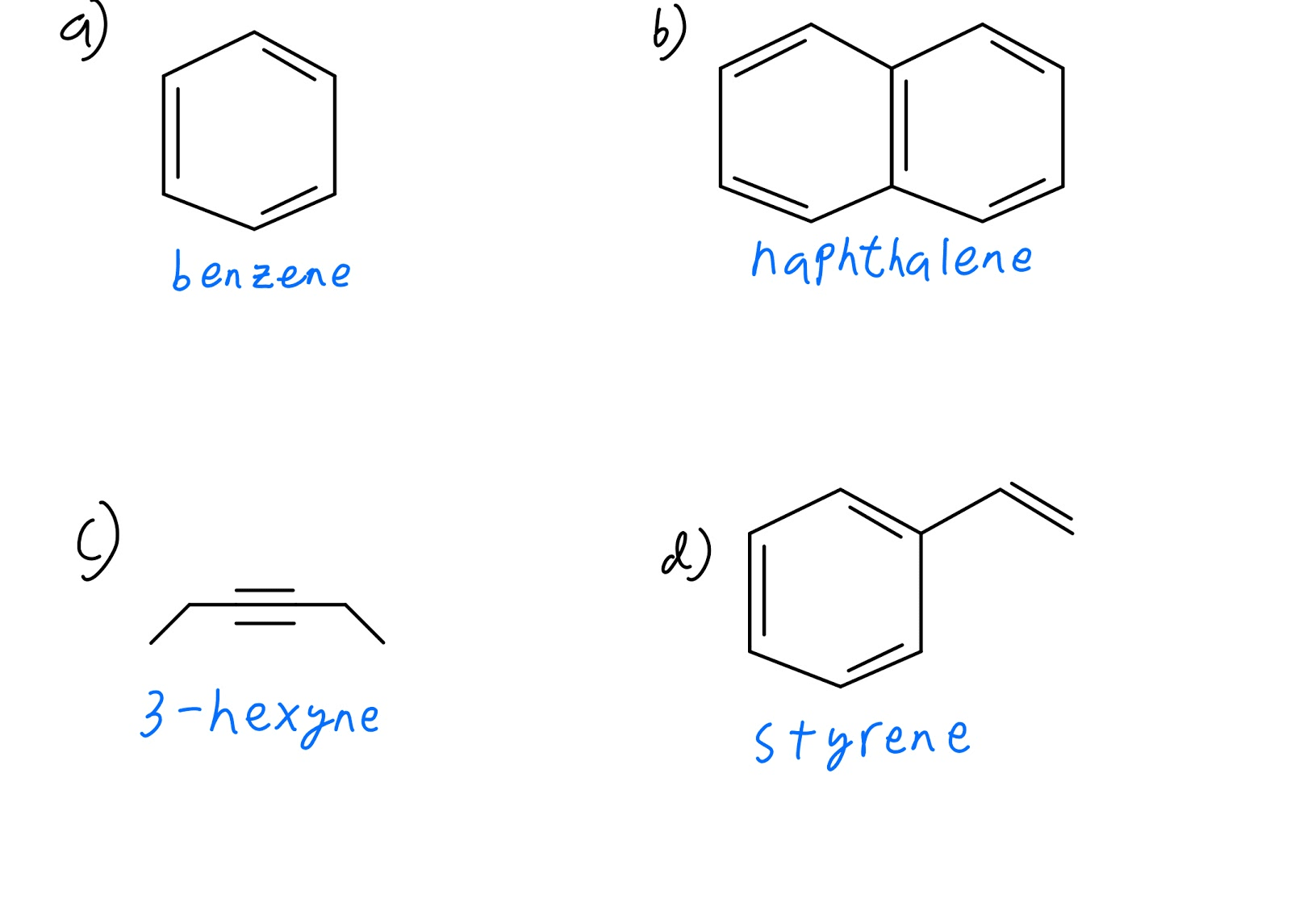
Ch3ch2ch2ch2ch2br 1 bromopentane 1 o ch3ch2ch2ch brch3 2 bromopentane 2 o ch3ch2ch brch2ch3 3 bromopentane 2 o ch32chch2ch2br 1 bromo 3 methylbutane 1 o ch32chchbrch3. Give the molecular formula c3h7br draw all the structural isomers that are possible.

Draw All The Structural Isomers For The Molecular Formula

Draw All The Structural Isomers For The Molecular Formula
Chapter 4 Lecture Notes Review The Octet Rule In The
Hey im working on this prelab and i cant figure out how many structural isomers exist for c3h7br.
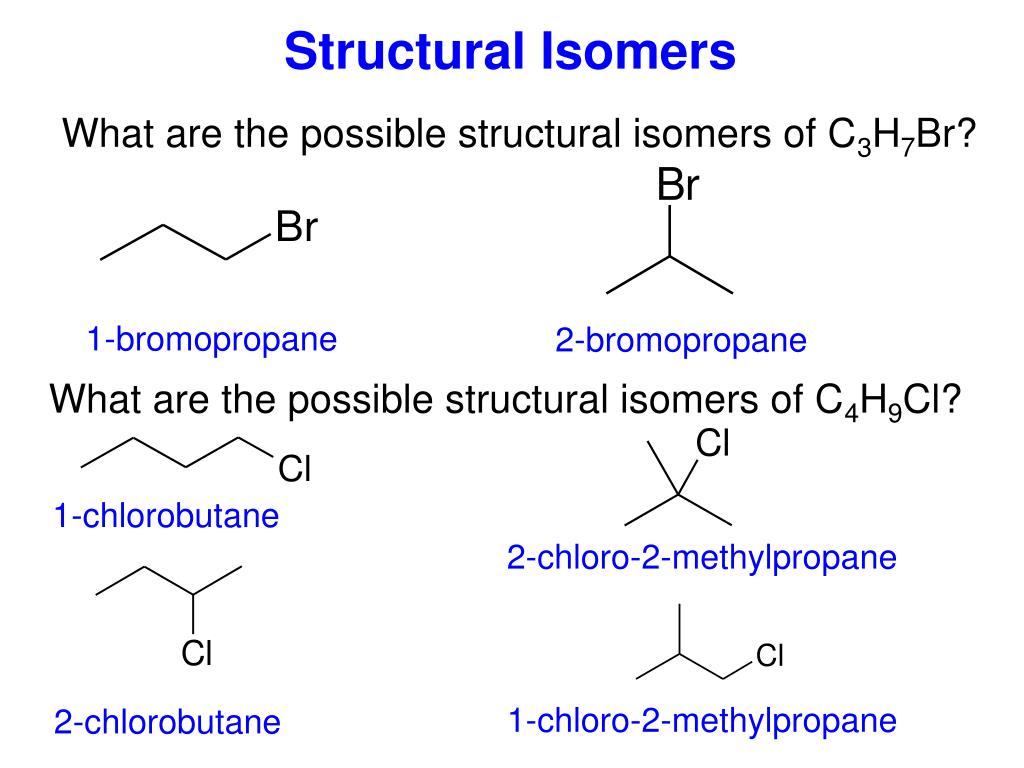
Draw all the structural isomers for the molecular formula c3h7br.
Draw the structures of all the eight structural isomers.
Drawing isomers from a molecular formula.
Name each isomer according to iupac system and classify them as primary secondary or tertiary bromide.
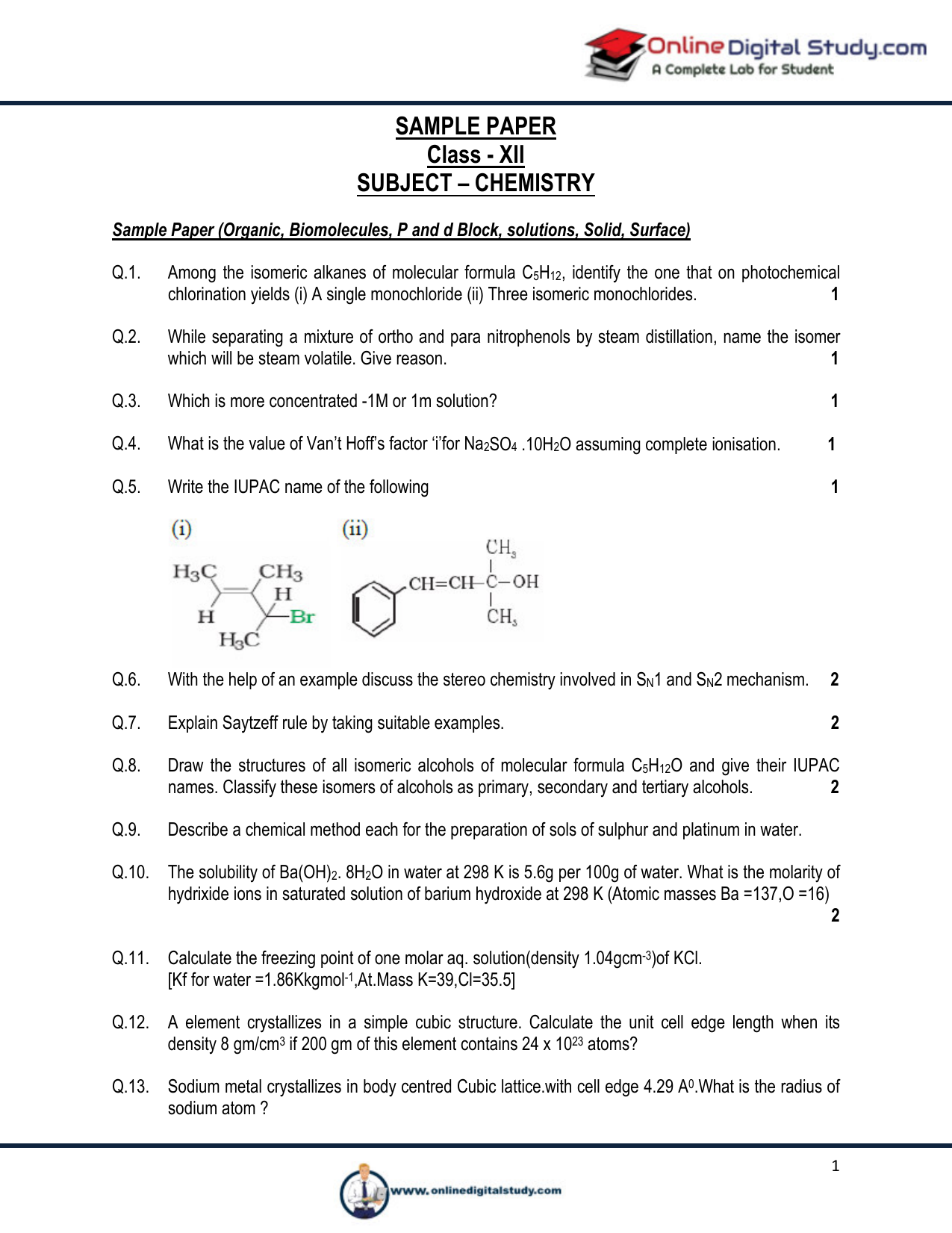
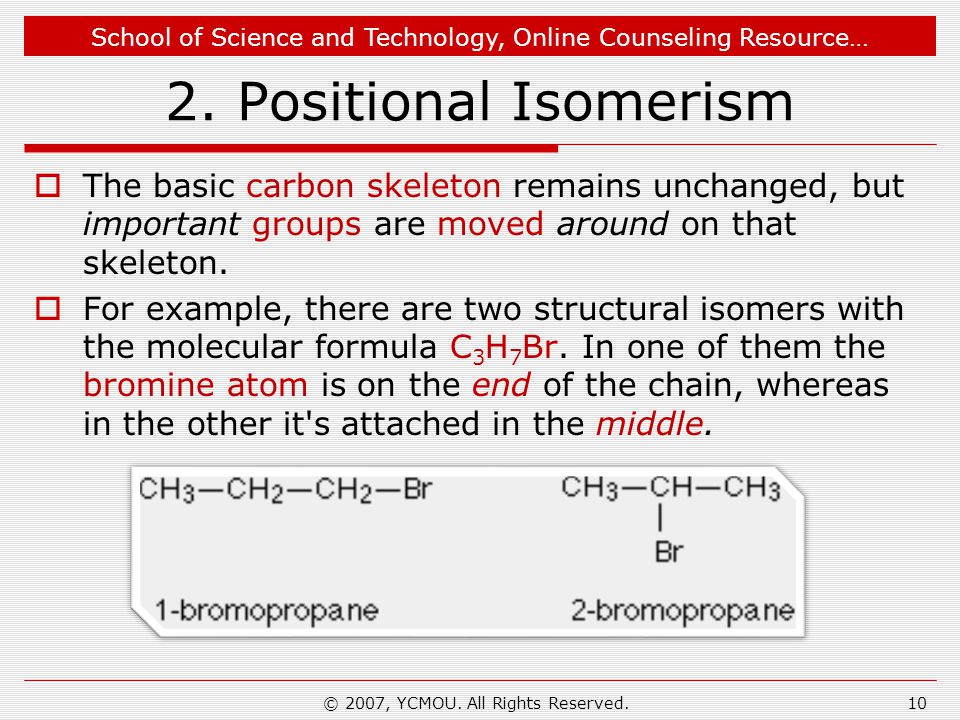
For example there are two structural isomers with the molecular formula c3h7br.
Structural isomers for butane having formula c4h10 are two.
Draw all the structural isomers for the molecular formula c3h7br.
Answer to give the molecular formula c3h7br draw all the structural isomers that are possible.
Draw all the structural isomers for the molecular formula c3h7br.
Draw all the structural isomers for the molecular formula c3h7br.
Draw the main chain minus 2 carbons and add two one carbon groups two methyls or one 2 carbon group an ethyl to as many positions possible trying not to repeat structures.
Structural isomers have same molecular formula but different structural formula.
If you made a model there is no way that you could twist one molecule to turn it into the other one.
One is n butane and the other is iso butane.
Give the molecular formula c3h7br draw all the structural isomers that are possible.
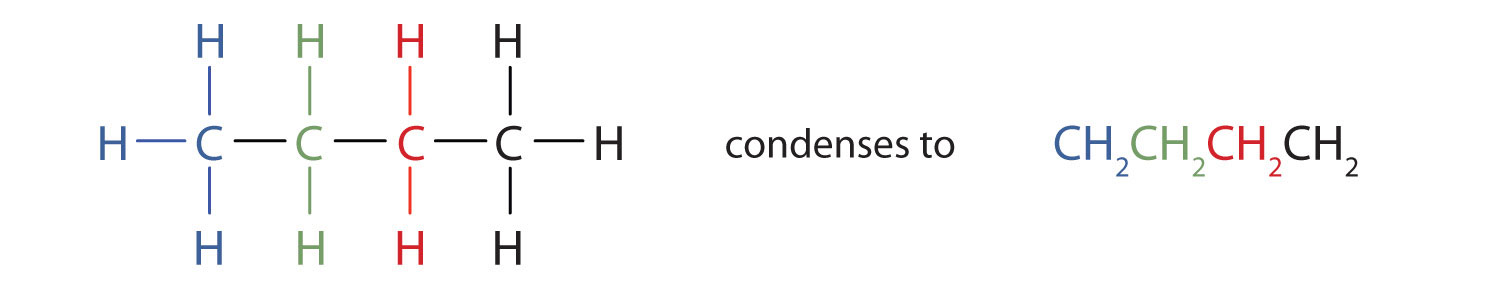
You can draw a full structural formula for each of them but we also can simply group the atoms to show.
The molecular formula c3h6o obviously does not show the difference between the isomers.
This problem has been solved.
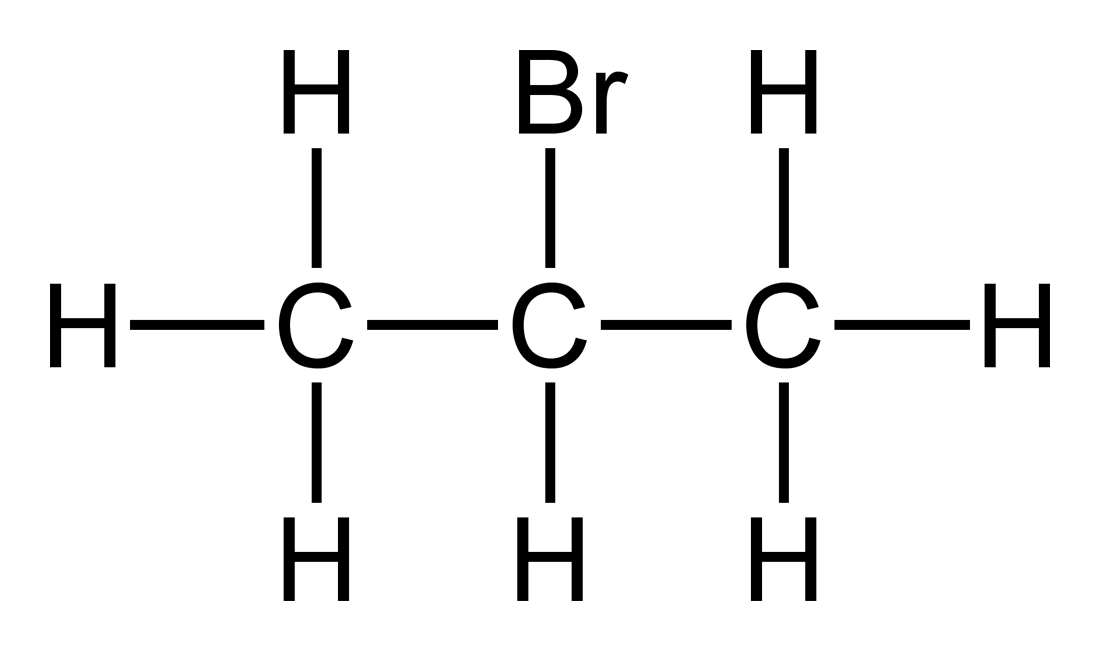
In one of them the bromine atom is on the end of the chain whereas in the other its attached in the middle.
Continue subtracting and adding groups in this fashion until you run out of carbons or doing so only results in repeated structures.
Answer to given the molecular formula c3h7br draw all the structural isomers that are possible.
Online Counseling Resource Ycmou Elearning Drive Ppt
Structural Isomerism Chain Positional Functional Group
Organic Chemistry Organic Chemistry The Chemistry Of Carbon

Isomers Definition Types Examples Video Lesson

Trick To Draw Find Total Possible Number Of Isomers For Alkanes
Solved Draw All The Structural Isomers For The Molecular
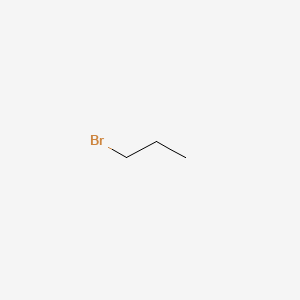
1 Bromopropane C3h7br Pubchem

3 4 Isomers Organic Chemistry 1 An Open Textbook

Test Bank For Organic Chemistry 8th Edition By Wade By

Draw All The Structural Isomers For The Molecular Formula
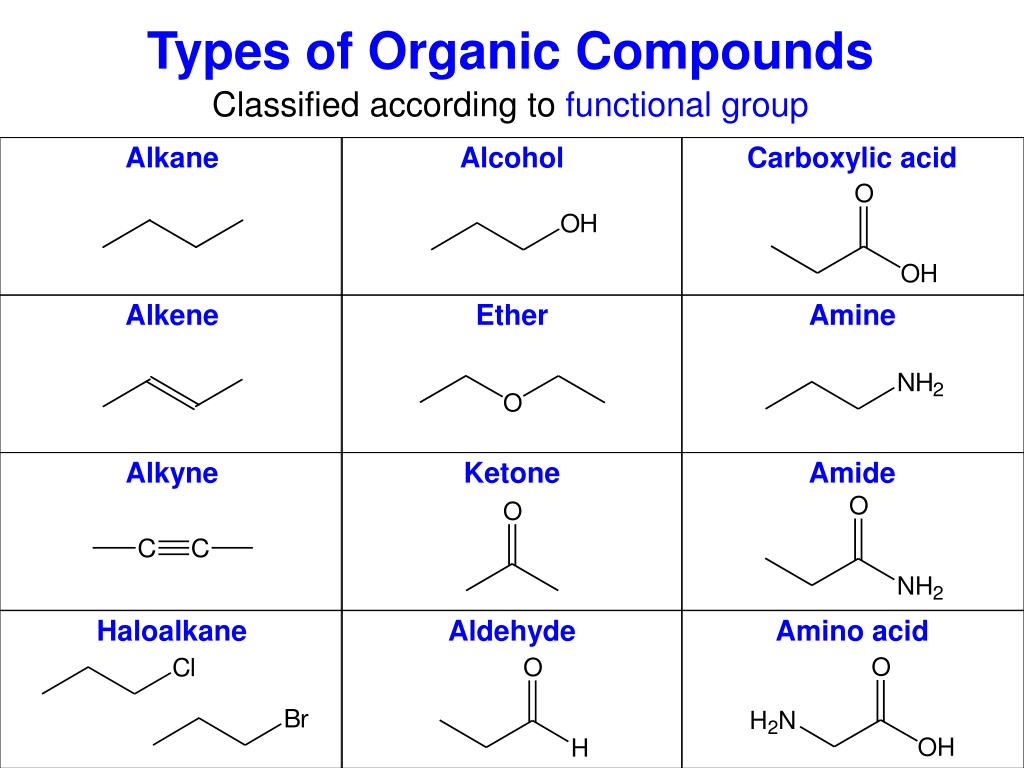
Ppt Organic Chemistry Powerpoint Presentation Free
Structural Isomerism In Organic Molecules Chemistry Libretexts
C3h7cl Structural Isomers Of C3h7br
Introduction To Organic Chemistry Alkanes
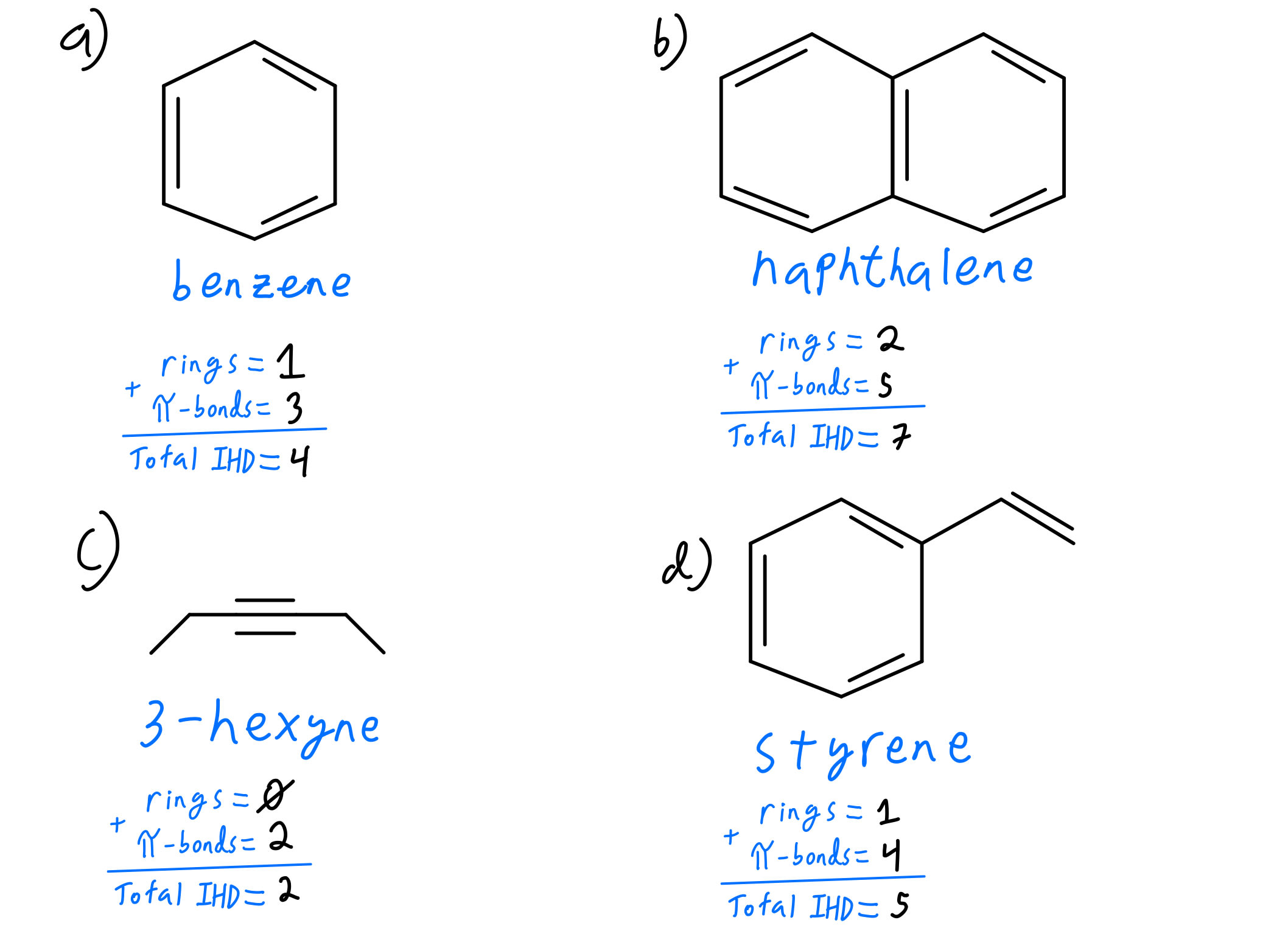
Degrees Of Unsaturation Organic Chemistry Video Clutch Prep
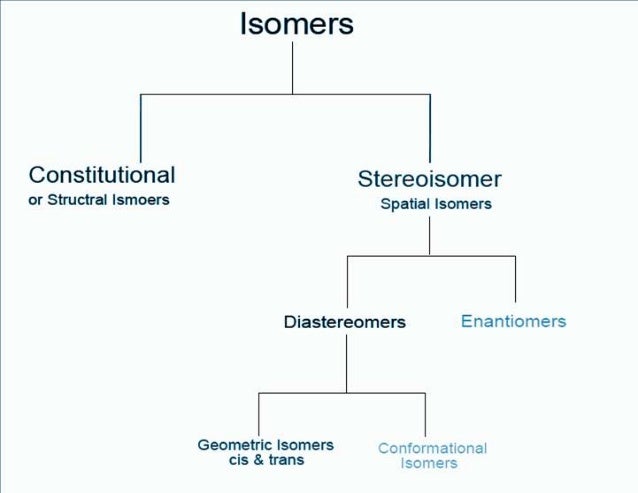
Isomers

Sample Paper Class Xii Subject
Organic Chemistry Assignment 1 Virtual High School Vhs
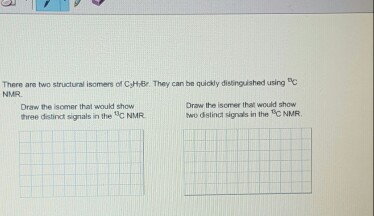
13cnmrq
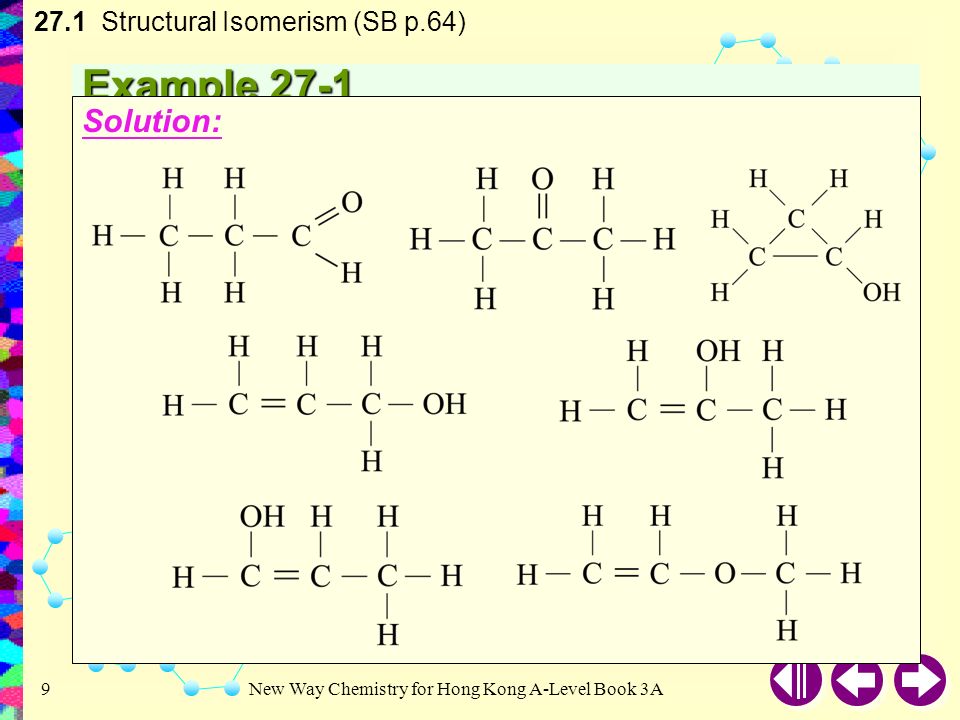
Chapter 27 Isomerism 27 1 Structural Isomerism 27 2

Isomerism Wa Ying College

3 4 Isomers Organic Chemistry 1 An Open Textbook
What Are Structural Isomers Give Me An Example Socratic
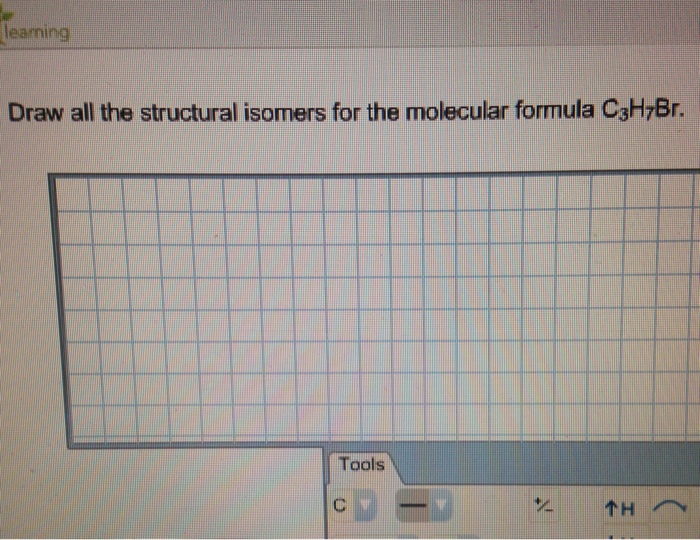
Solved Learning Draw All The Structural Isomers For The M

Convert This Molecular Formula Into A Structure That Is
Degrees Of Unsaturation Organic Chemistry Video Clutch Prep
Draw All The Structural Isomers For The Molecular Formula

Organic Problems
Draw All The Structural Isomers For The Molecular Formula

Condensed Structural And Line Angle Formulas

Organic Chemistry Organic Chemistry The Chemistry Of Carbon
Unit 13 Organic Chemistry
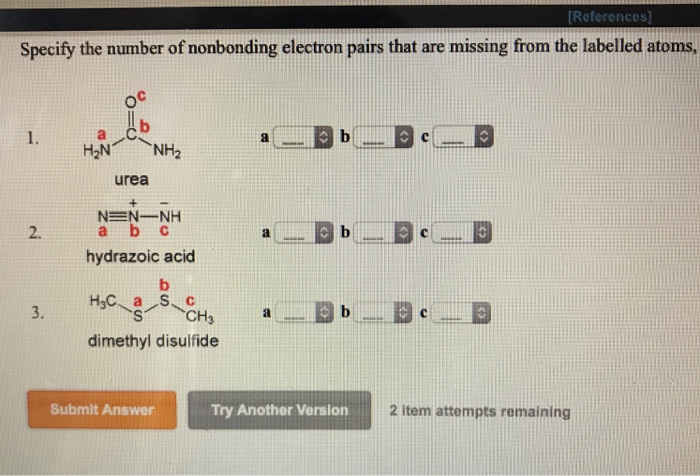
Solved Convert This Molecular Formula Into A Structure Th
Ppt Organic Chemistry Powerpoint Presentation Free
Structural Isomerism In Organic Molecules Chemistry Libretexts

1 Q What Are Saturated And Unsaturated Hydrocarbons Imp

Sch4u Organic Chemistry Assignment 1 Docx Sch4u Organic
Condensed Structural And Line Angle Formulas
No comments:
Post a Comment