If the difference is zero the bond is considered nonpolar covalent. For the n3 lewis structure calculate the total number of valence electrons for the n3 molecule.

Draw The Lewis Structure Of Clf3 Clutch Prep

Note Webassign Is Looking For Numbers Not Words Or Fractions

Draw The Main Lewis Structure Of Nof Draw Nonbonding Electrons Using The Dot Notation And Bonding Electrons As A Bond Determine The Number Of Bonding Electrons And The Number Of Nonbonding Electrons
After drawing a lewis structure one should a.
Draw the main lewis structure of nof if n is the central atom in the molecule.
In the lewis structure for nof there are a total of 18 valence electrons.
Draw nonbonding electrons using the dot notation and bonding electrons as a bond.
Nitrogen n is the least electronegative element in the nof lewis structure and therefore goes in the center of the structure.
List three properties of a covalent compound.
The hybridization of the central atom al in albr3 is a.
Confirm that the total number of valence electrons used equals the number available d.
The solid state of a molecule is crystalline.
If greater than 17 ionic.
Draw the main lewis structure of nof.
Be sure to use the number of available valence electrons you found.
The nof lewis structure is very similar to nocl and nobr.
Determine the electronegativity of each atom.
Consider the skeletal structure shown below.
Determine the number of bonding electrons and the number of nonbonding electrons in the structure of bef2.
Nccn draw the lewis structure and answer the following.
The lewis structure for nof has a total of 18 valence electrons.
In the nof lewis structure nitrogen n is the least electronegative atom and goes in the center of the lewis structure.
The line indicates the bond between s and cl and.
Determine the number of each type of atom in the molecule b.
Drawing the lewis structure for nof.
Determine the number of bonding electrons and the number of nonbonding electrons in the structure of scl2.
If between zero and 17 polar covalent.
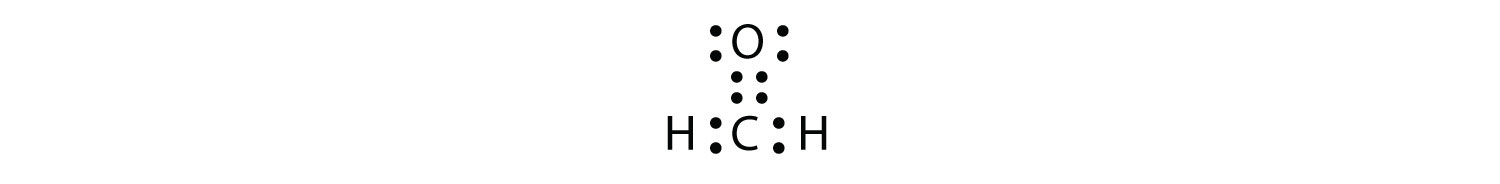
The bond angles about the carbon atom in the formaldehyde molecule h2co are about.
The electronegativity of an element is its propensity to attract electrons and the element in a compound with the lowest electronegativity is usually the central one.
Add unshared pairs of electrons c.
Check the formal charges to be sure that each atom has a formal charge of zero.
Draw the main lewis structure of nof.
The exception to this rule is hydrogen which is never the central atom except in the h2 molecule.
After determining how many valence electrons there are in n3 place them around the central atom to complete the octets.
Comparing electronegativity is the most reliable way to determine the central atom.
In the lewis structure for n3 youll need to place a double bonds between the nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule.
A molecular solid is a nonconductor is soft and has a low melting point.

Molecular Geometry Ck 12 Foundation
Molecular Shapes

1 8 Chemistry 2810 Answers To Assignment 3 Topic Lewis

Lewis Dot Structure Chemistry Video Clutch Prep

How Would You Draw All The Resonance Structures For Nitrate
Molecular Shapes
Molecular Orbital Theory
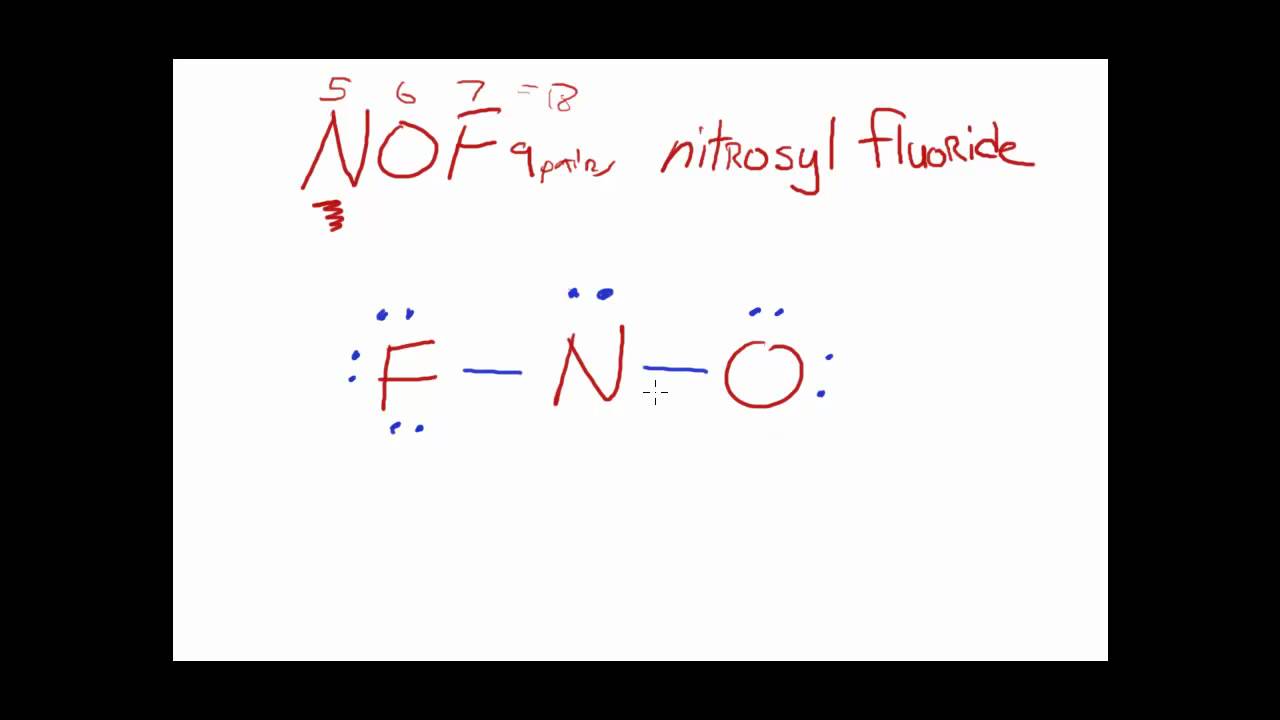
Structure And Geometry The Nof Example
How To Tell If A Molecule Is Polar Or Non Polar Vsepr

Chemistry Chap 4 Flashcards Quizlet
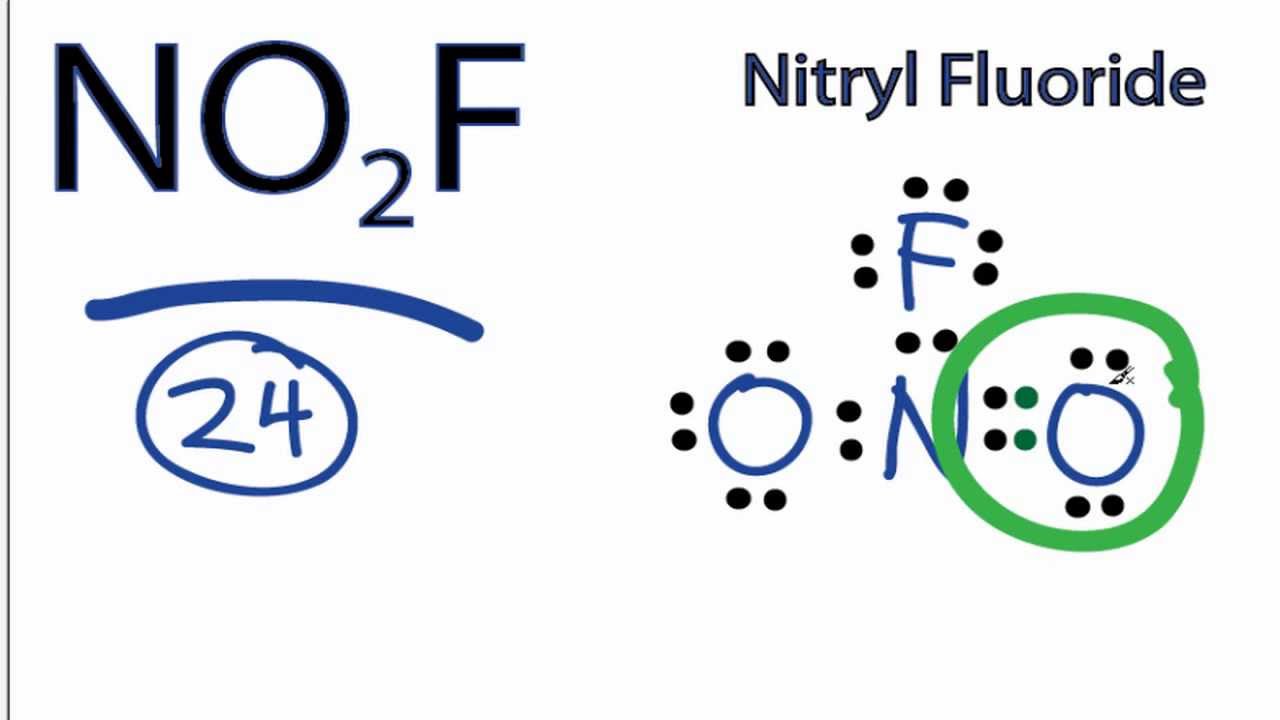
No2f Lewis Structure How To Draw The Lewis Structure For No2f
Ch 3 Understanding Diagrams

Note Webassign Is Looking For Numbers Not Words Or Fractions
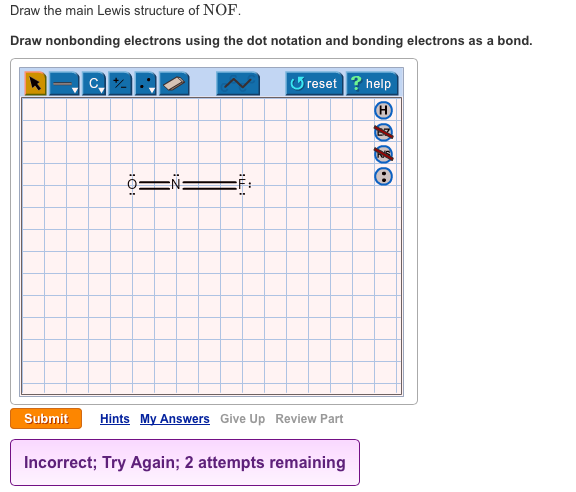
Solved Draw The Main Lewis Structure Of Nof Draw Nonbond
2018 G11 Chemistry E Pages 301 350 Text Version Pubhtml5
Chem1000a Spring 2007 Practice Assignment 6 Answers

Ambivalent Lewis Acid Bases With Symmetry Signatures And

So2 Sulfur Dioxide Molecular Geometry Lewis Structure
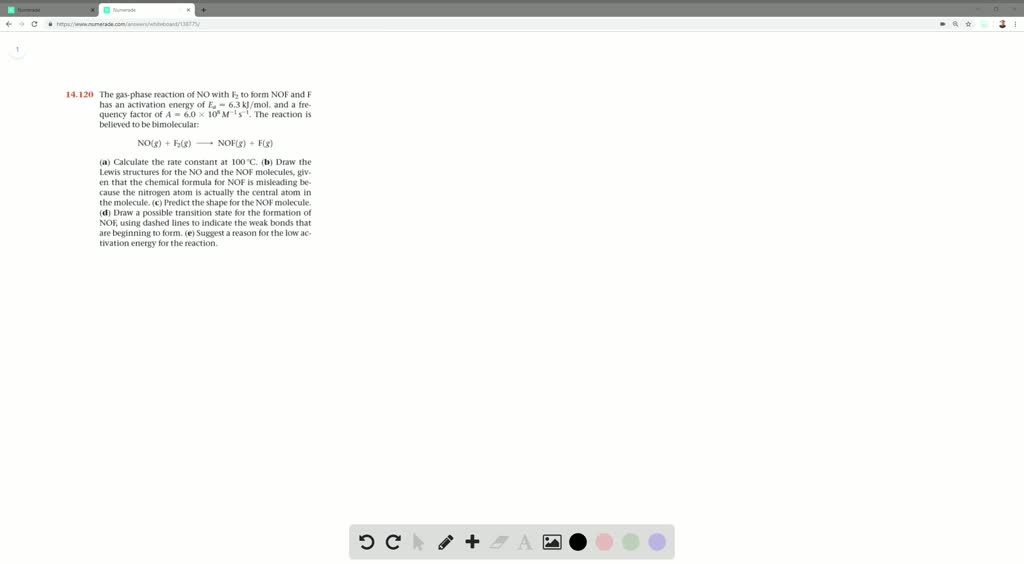
The Gas Phase Reaction Of No With Mathrmf 2 To Form Mathrmnof And Mathrmf Has An Activation Energy O
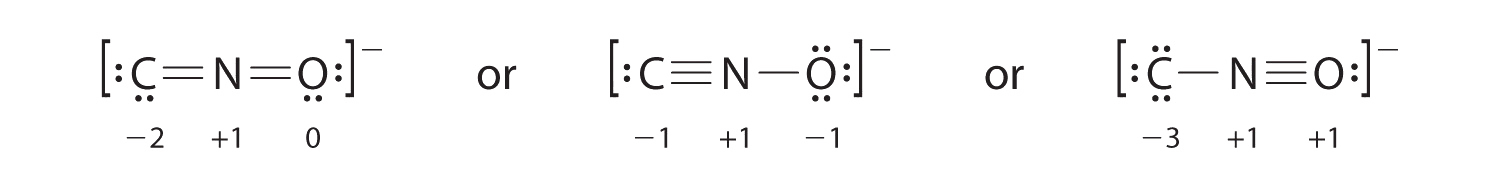
8 5 Drawing Lewis Structures Chemistry Libretexts

General Chemistry Nitrogen Hydrogen Peroxide

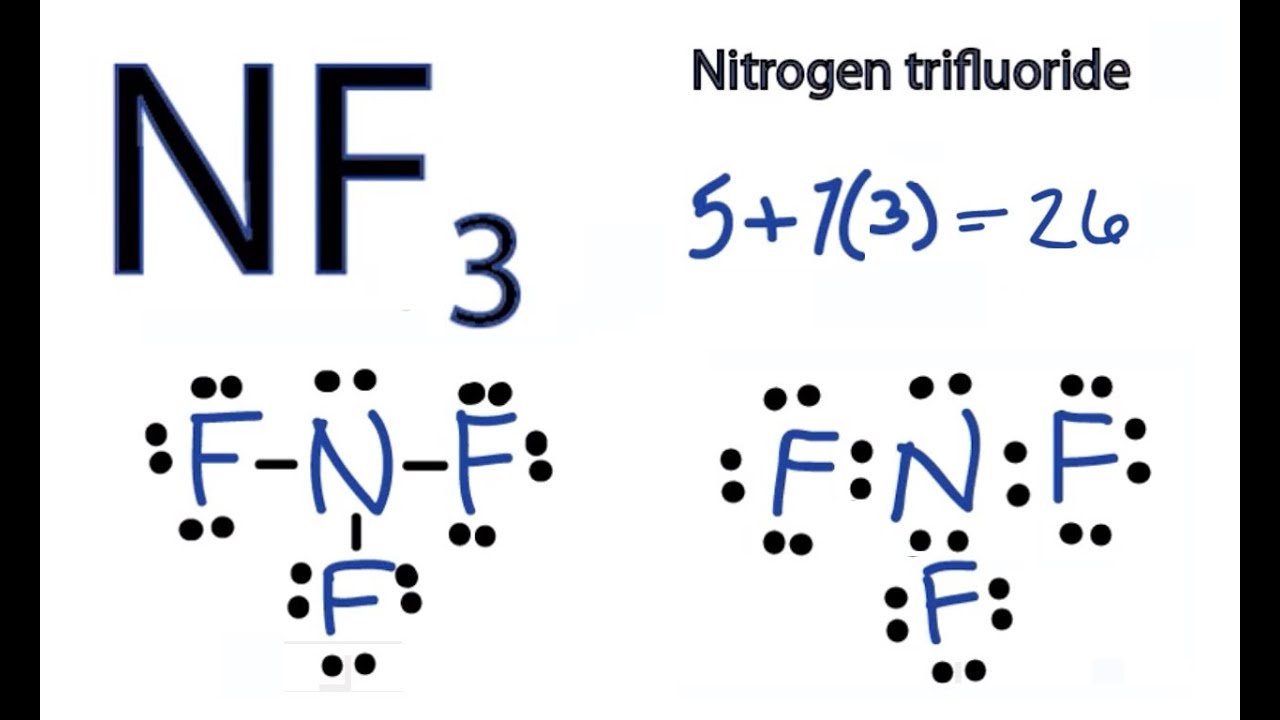
Nf3 Lewis Structure How To Draw The Dot Structure For Nf3 Nitrogen Trifluoride
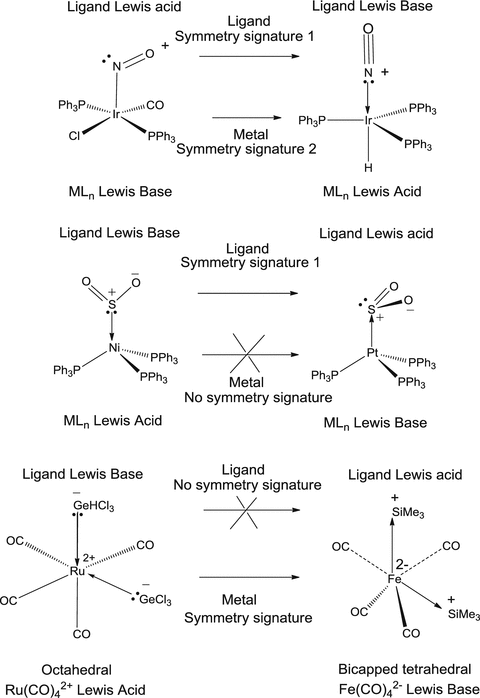
Ambivalent Lewis Acid Bases With Symmetry Signatures And

Nof Lewis Structure How To Draw The Lewis Structure For Nof
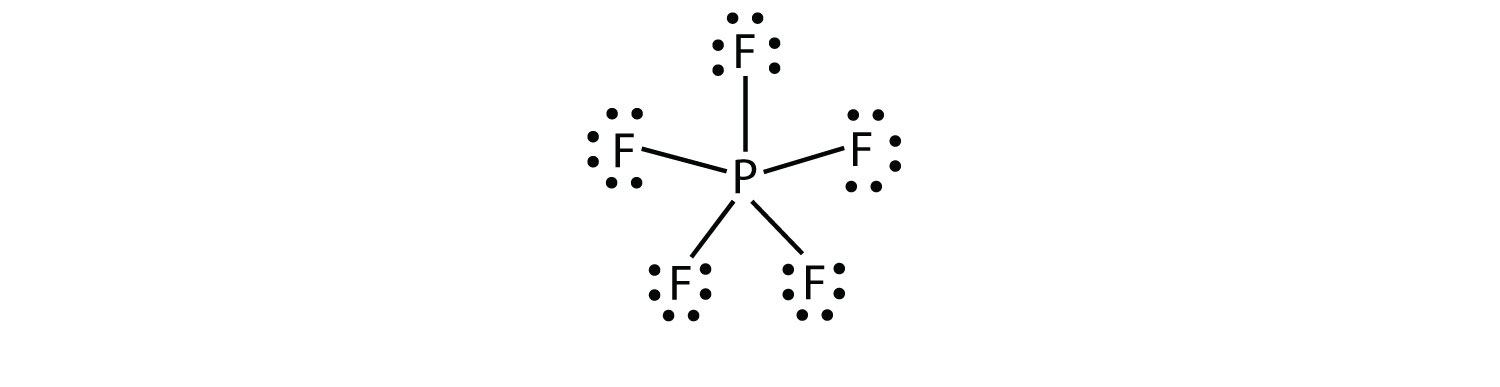
Chapter 8 Chemical Bonds Che 105 110 Introduction To

Solutions Manual For Chemical Principles The Quest For

Ppt Homework Problems Powerpoint Presentation Free

What Would The Lewis Structure Of Nof Look Like Yahoo Answers

Molecular And Ionic Compounds Chemistry For Majors
Lewis Structure Drawing Questions

Steric Number A Focus Into The Key To The Vsepr Theory

Hno3 Lewis Structure How To Draw The Lewis Structure For Hno3

Masteringchemistry Drawing Lewis Structures Youtube
Molecular Orbital Theory
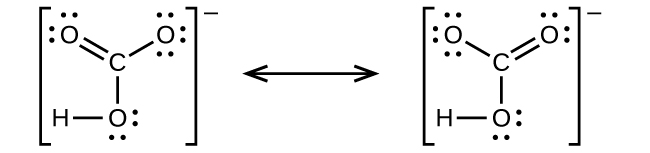
7 4 Formal Charges And Resonance Chemistry
Solutions Manual For Chemical Principles The Quest For

Vsepr And Bond Angles

Draw The Lewis Structure For Acetamide Ch Clutch Prep
No comments:
Post a Comment